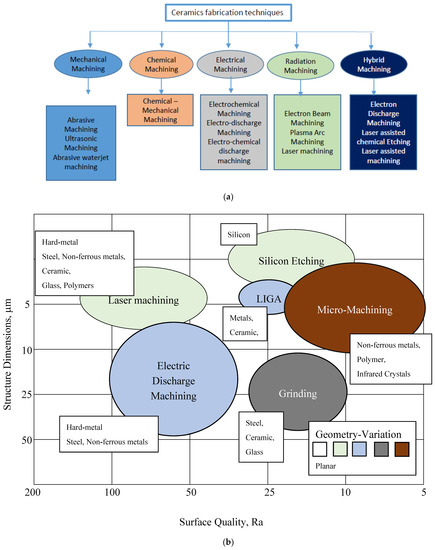
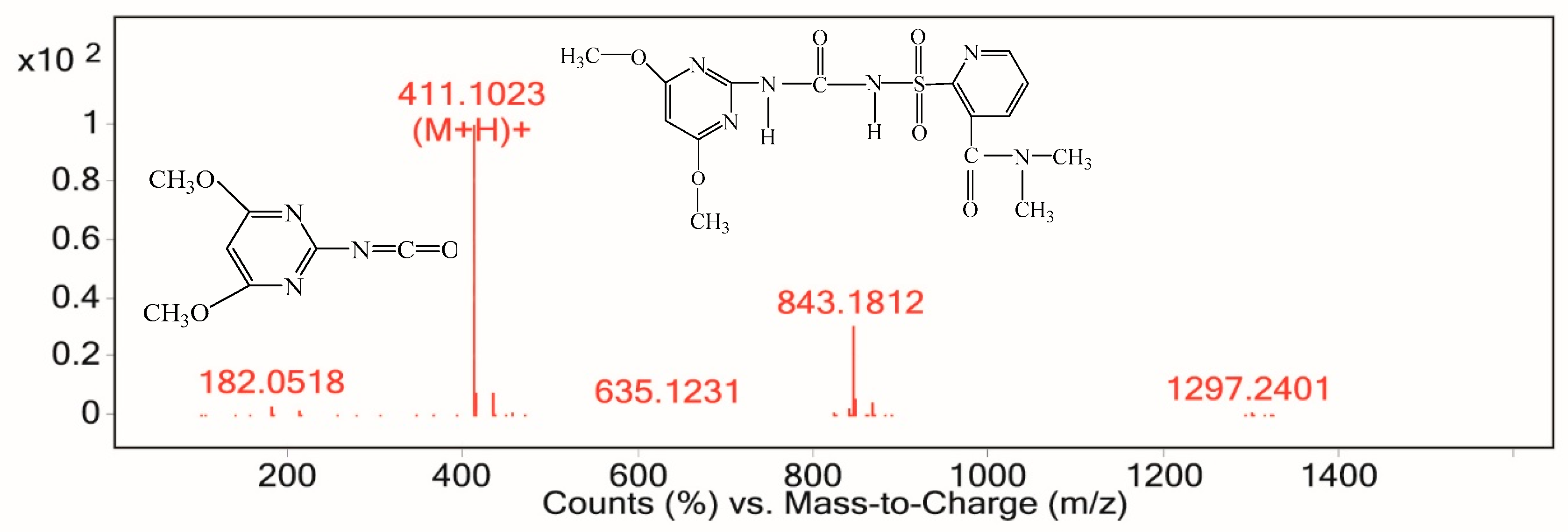
Ceramics Electrochemical Degradation

Ceramics which are not susceptible to electrochemical degradation due to its poor con ductor property but due to the simple dissolution of the material.
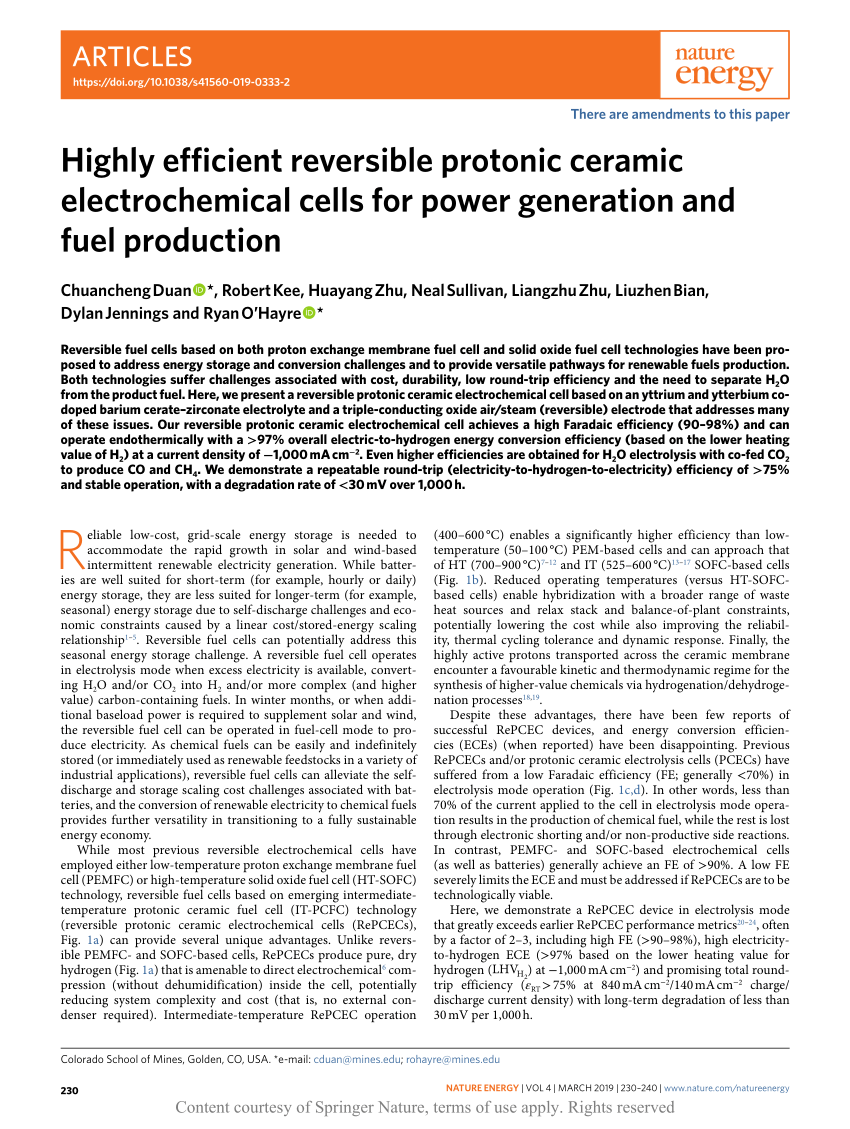
Ceramics electrochemical degradation. Water induced degradation of barium titanate bto ceramics has been investigated using electrochemical hydrogen charging in which the silver electrodes of bto ceramic specimens are made cathodes in a 0 01m naoh solution to evolve hydrogen by electrolysis of water. Artificial saliva was used as a degradation medium to assess the effect of saliva on the surface condition microstructure chemical composition and degradation behavior of dental materials. Near complete removal 98 30 0 51 of pfos was achieved under a cross flow filtration mode at the anodic potential of 3 15 v vs standard. While the observed pcfc scalability and electrochemical performance are encouraging protonic ceramic stack degradation has witnessed limited study.
Deterioration at high. 2fe o 2 æ2feo. We observed acute performance degradation in our initial stacks with galvanostatic voltage degradation exceeding 100 kh 1 under hydrogen air reactants at 550 c. The process of an oxide layer formation is an electrochemical process.
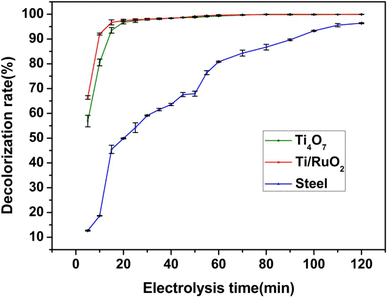
The electrocatalytic stability of the ti 4 o 7 porous ceramic anode was also evaluated by a 10 day pfoa electrochemical degradation repeated test for details see text s5 and fig. In recent years there. This is done using x ray diffraction sem coupled energy dispersive spectrometry edx and electrochemical impedance spectroscopy of these ceramics before and after 21 days of immersion in the salivary. Jacers is a leading source for top quality basic science research and modeling spanning the diverse field of ceramic and glass materials science.
This study investigated the degradation of perfluorooctanesulfonate pfos in a reactive electrochemical membrane rem system in which a porous magnéli phase titanium suboxide ceramic membrane served simultaneously as the anode and the membrane. In the case of iron heated to high temperature the reaction is as follows. Calculations of oxygen chemical potentials in the interior of ceramic electrodes with ion blocking interfaces indicate that phase changes and or decomposition will occur as a result of dc polarizat. Corrosion and degradation of materials.